Printable worldmap: Serialization track and trace deadlines
Add bookmark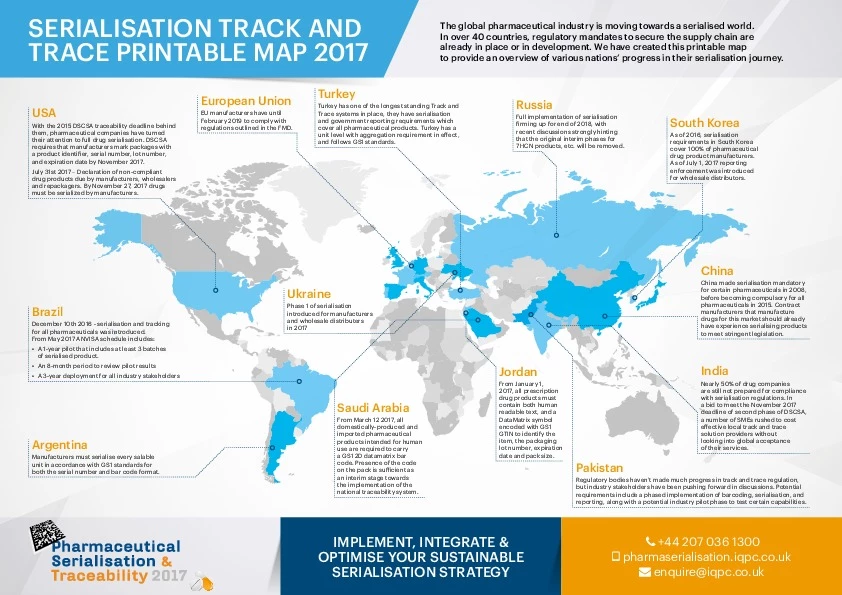
In over 40 countries, regulatory mandates to secure the pharma supply chain are already in place or in development.
Despite the urgency, many medicine manufacturers are known to be behind schedule with serialization projects. In our 2016 research a number of participants admitted to not even thinking about serialization at all as of yet.
 | Download this printable map to assist with your worldwide track and trace compliance strategies. |
Supply chain expert Tracelink predicts that as many as 400 contract manufacturing organizations (CMOs) will not be ready for the approaching US and EU serialization compliance deadlines. Tracelink estimates this equates to 50 per cent of the US’s and EU’s CMOs. This would leave many pharma firms without adequate support for serialization deadlines and, therefore, could spark a major shift in the CMO marketplace.
 |
The late adopters are going to be critically reliant on excellence in project execution to get their programmes in place and delivered on time. A high level of optimism was implied in last year’s results in regards to time left to complete serialization projects. With deadlines looming, instead of being complacent the industry should utilize the time left to avoid any costly mistakes.
The more prepared manufacturers are assessing the returns available from the legal requirements. More than 70 per cent of manufacturers, in a recent industry study commissioned by KPMG, appreciated that serialization requirements will have a positive impact on their business processes.
Ahead of the 2018 Serialization and Traceability event, We have created this map to provide an overview of various nations’ progress in their serialization journey.
[inlinead]

















